Utility of clinical exome sequencing in a complex Emirati pediatric cohort.
Clinical exome sequencing (CES) has develop into a routine diagnostic software in a number of pediatric subspecialties, with a reported common diagnostic yield of ~25% in this affected person poulation.
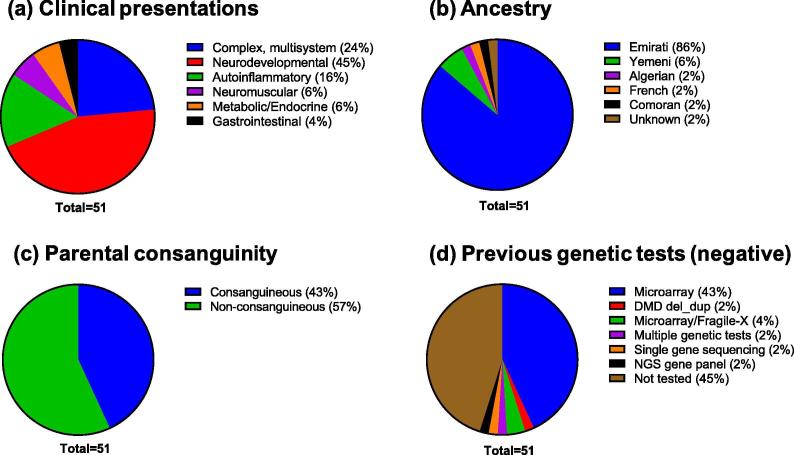
The utility of CES in the United Arab Emirates (UAE) has not been beforehand investigated, most certainly as a result of lack of the suitable tertiary pediatric facilities and diagnostic genomic services in this nation. Here, we report, for the primary time, CES findings on a multispecialty pediatric cohort in the UAE (N = 51). This cohort, which was largely Emirati (86%; 44/51), was adopted at Al Jalila Children’s Hospital (AJCH), the primary and solely devoted tertiary pediatric heart in the nation.
CES demonstrates a excessive diagnostic yield (41%; 21/51) in this cohort, the place 55% (28/51) had earlier non-diagnostic genetic testing whereas for the remaining people (45%), CES was the first-tier take a look at. Given the reported excessive consanguinity fee in this inhabitants, 48% of the constructive instances (10/21) have been resulting from genes related to recessive circumstances.
However, 11 out of 21 constructive instances (52%) have been resulting from heterozygous pathogenic variants in genes identified to trigger dominantly inherited issues, together with a case with a twin analysis attributed to 2 completely different genes (2%; 1/51), and one other case with a novel de novo variant and new phenotypic options for a identified gene (2%; 1/51). Overall, we now have recognized 13 novel clinically vital variants and confirmed that software of CES as a first-tier take a look at performs a vital position in genetic analysis and administration of Emirati pediatric sufferers.
Understanding the Barriers to Pediatric Oncologist Engagement and Accrual to Clinical Trials in National Cancer Institute-Designated Community Oncology Research Programs.
Clinical trial participation results in progress in most cancers care. Principal investigators (PIs) and clinical analysis associates (CRAs) play key roles in the availability and upkeep of clinical trial portfolios at their websites.
Previous research have evaluated the academic and useful resource wants of grownup oncology suppliers, however nothing thus far has targeted on suppliers of pediatric oncology care.
We aimed to establish the academic wants and clinical trial participation limitations at National Cancer Institute Community Oncology Research Program (NCORP) Children’s Oncology Group (COG) websites to enhance the standard of website investigator engagement.Quality enchancment surveys of pediatric clinical analysis employees at NCORP websites have been carried out.
The first was a web-based inquiry of NCORP COG PIs and lead CRAs to evaluate their common understanding of NCORP organizational construction and desires. The second survey of COG PIs was carried out by one-on-one phone interviews aimed toward figuring out particular limitations to doctor engagement and affected person enrollment in clinical trial analysis.
The majority of NCORP COG PIs and CRAs (63%) reported an incomplete understanding of NCORP construction, with roughly half expressing curiosity in creating stronger collaborations and engagement.
Most NCORP COG PIs reported a minimum of one shared barrier to clinical trial enrollment (78%), with insufficient protected time and analysis assist (39% every) being essentially the most often cited limitations.Contributions to pediatric most cancers clinical analysis at COG NCORP websites might be enhanced by means of improved training, sources, and time allocation.